Does Sds Page Break Disulfide Bonds - In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.
Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures;
Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures;
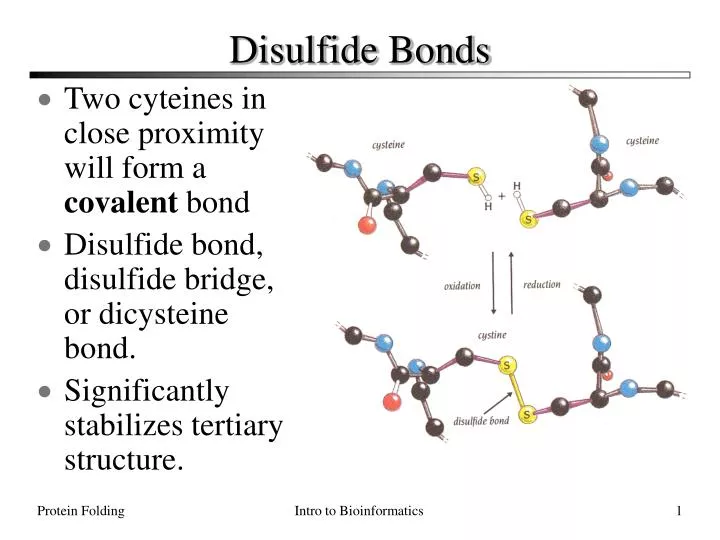
PPT Disulfide Bonds PowerPoint Presentation ID165240
Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme.
Disulfide bond wikidoc
In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.
Figure SI 6 Effect of disulfide bonds on HdeA variants' migration in
Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures;
SOLVED If you wanted to break disulfide bonds in thick, tenacious
However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.
NonReducing SDS PAGE A Method to Analyze DisulfideLinked Multimeric
In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.
organic chemistry Can acidic conditions break disulfide bonds
However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.
The free sulfhydryl (a), disulfide bond contents (b), carbonyl contents
However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme.
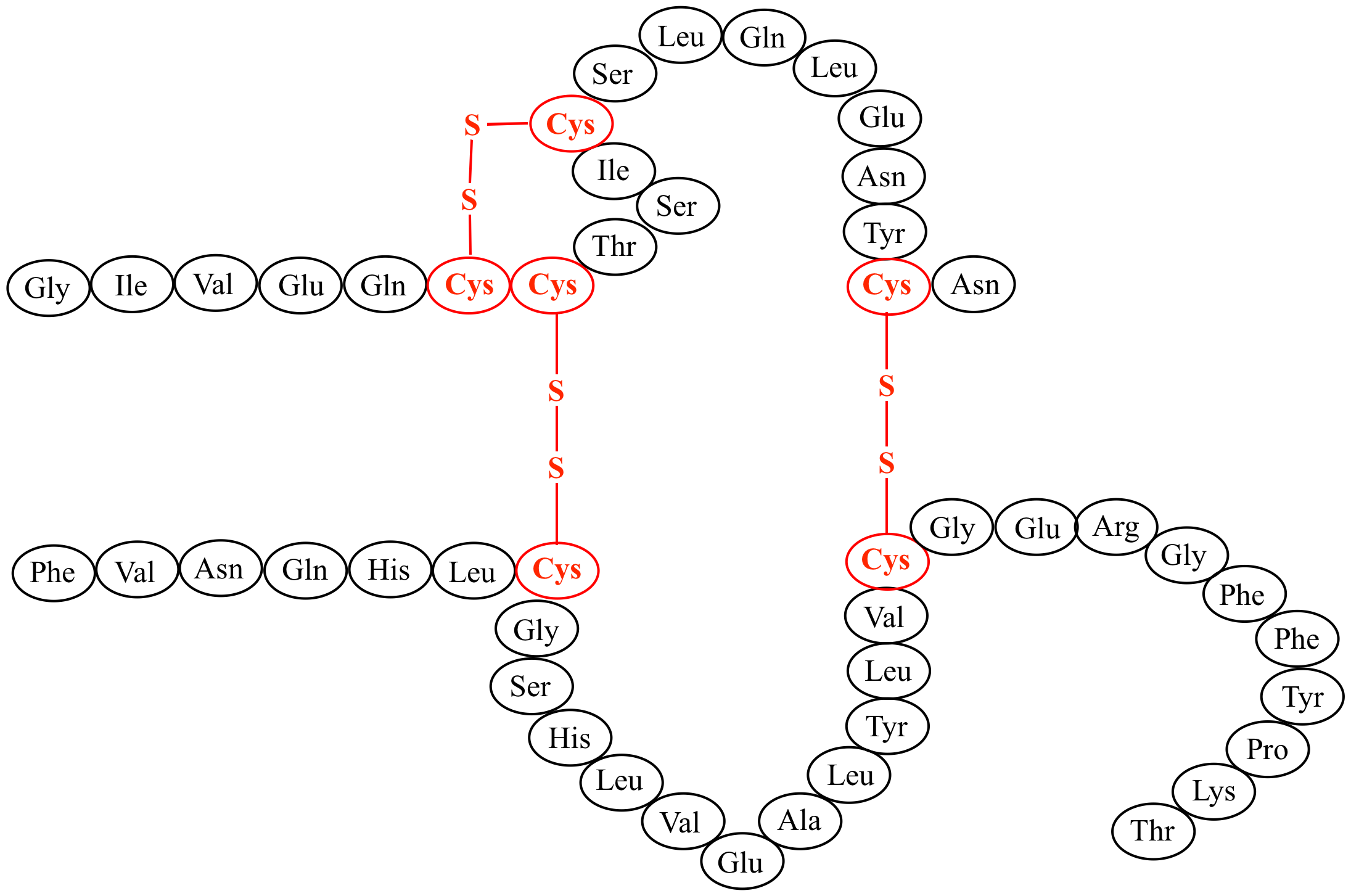
Interchain disulfide bonds are absent and intrachain disulfide bonds
Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme.
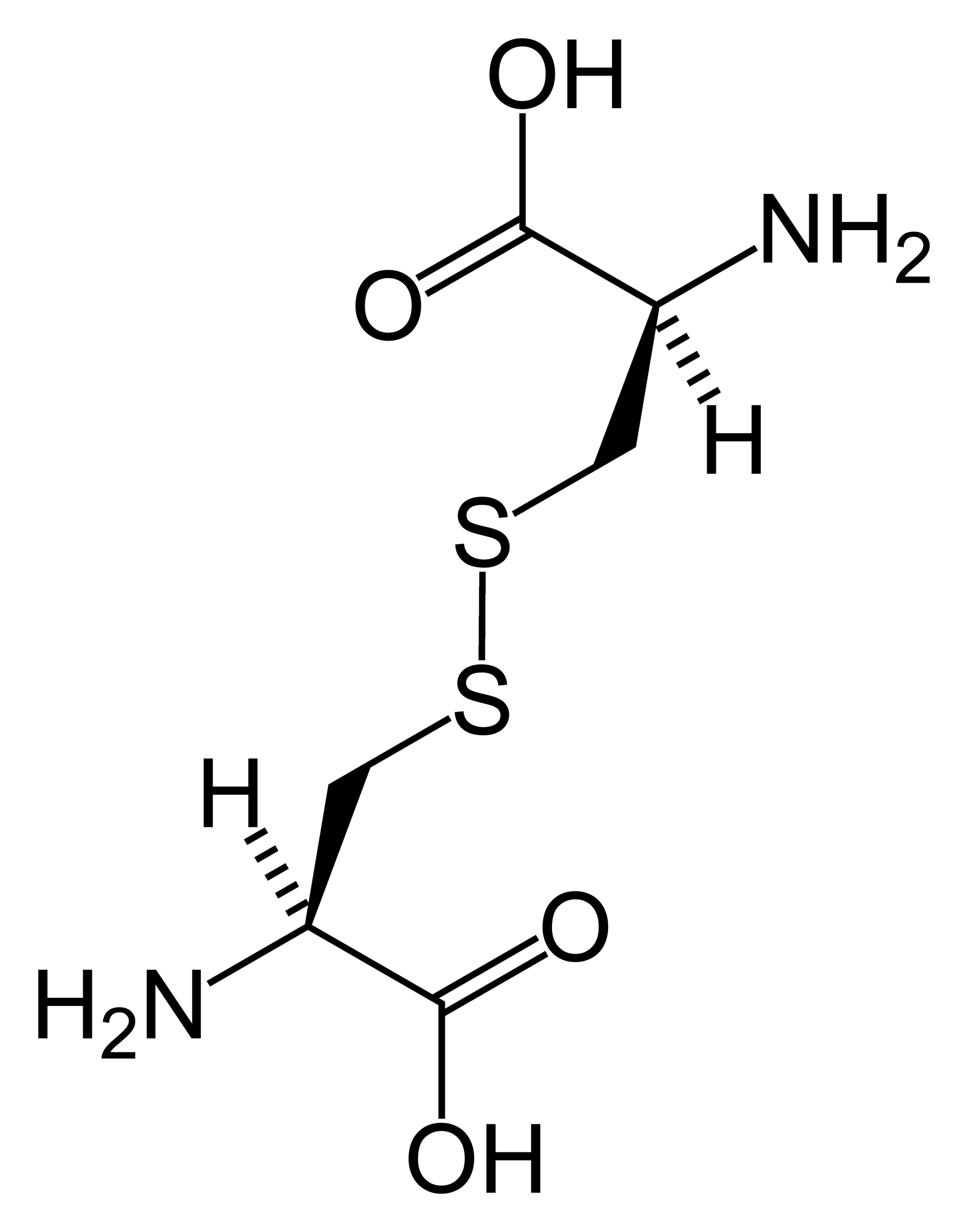
Illustrated Glossary of Organic Chemistry Disulfide bridge
Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents. In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures;
Analysis of TdaR, AclT and GliT on SDSPAGE (A) and enzymemediated
In summary, sodium dodecyl sulfate does indeed break disulfide bonds when combined with appropriate reducing agents like dtt or bme. However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.
In Summary, Sodium Dodecyl Sulfate Does Indeed Break Disulfide Bonds When Combined With Appropriate Reducing Agents Like Dtt Or Bme.
However, sds does not break down any of the disulfide bonds that participate in many tertiary structures; Sds does not break disulfide bonds because they are covalent bonds which are strong, so they need specific agents.