How Does Sds Page Separate Proteins - When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. It combines the denaturing properties of sodium.
When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. It combines the denaturing properties of sodium. Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β.
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. It combines the denaturing properties of sodium.
How Does Sds Page Separate Proteins Metro Cooking Dallas
It combines the denaturing properties of sodium. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β.
LearnSci LabSim SDSPAGE Analysis
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. It combines the denaturing properties of sodium.
Solved Why is SDSPAGE not used to separate proteins? Select
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. It combines the denaturing properties of sodium. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively.
How Does SDS Denature Proteins
It combines the denaturing properties of sodium. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β.
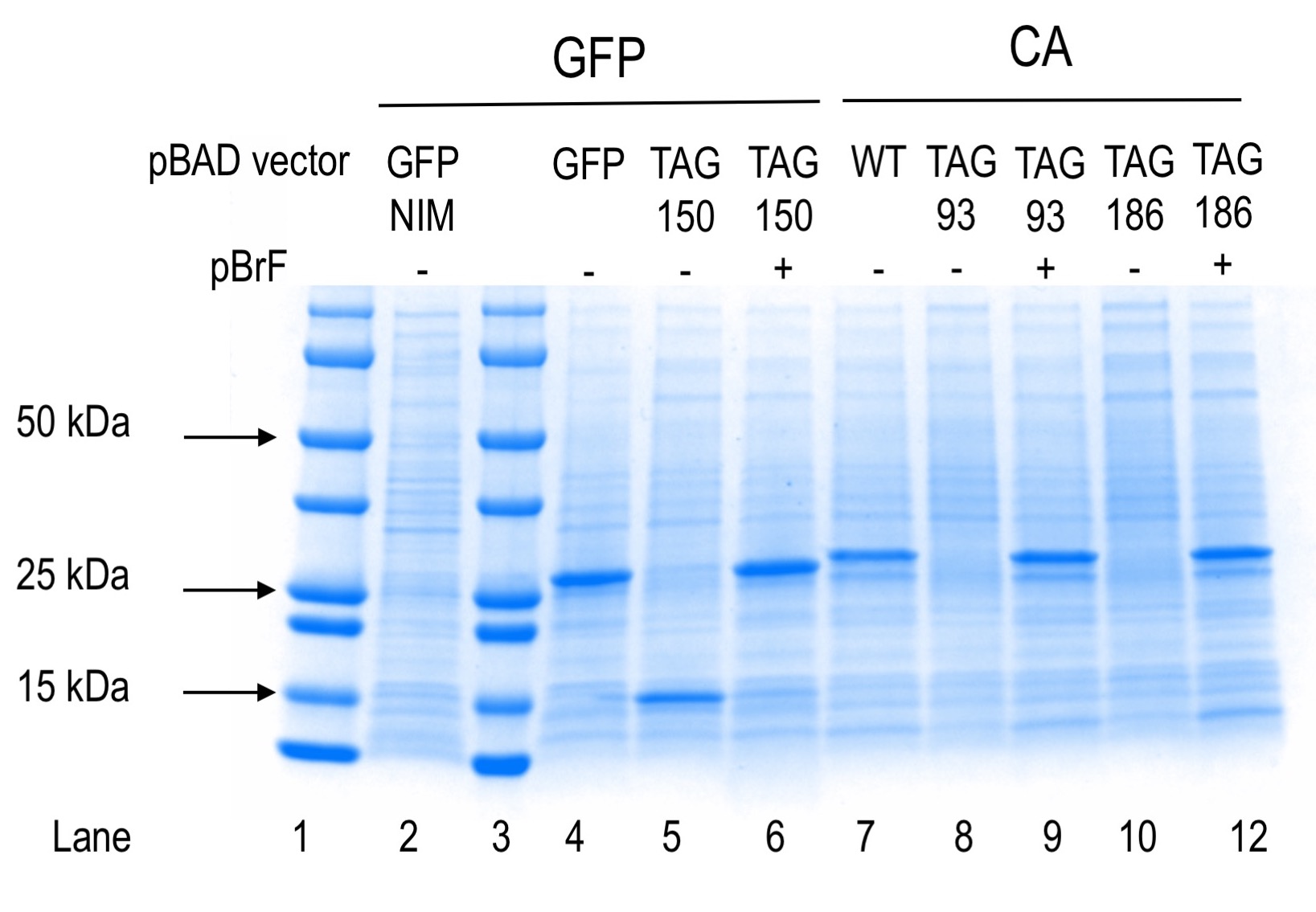
Proteins are separated in an SDSPAGE experiment on the basis of their
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. It combines the denaturing properties of sodium.
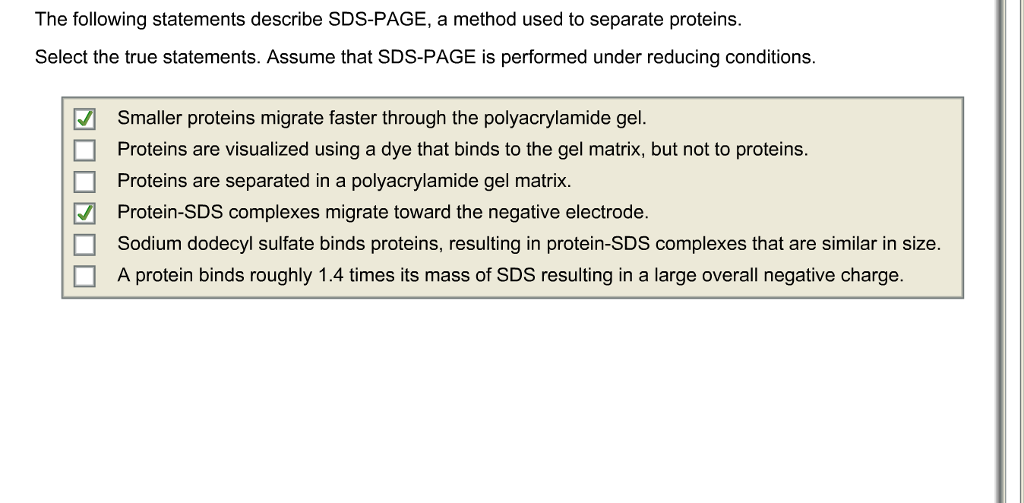
Solved The following statements describe SDSPAGE, a method
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. It combines the denaturing properties of sodium. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively.
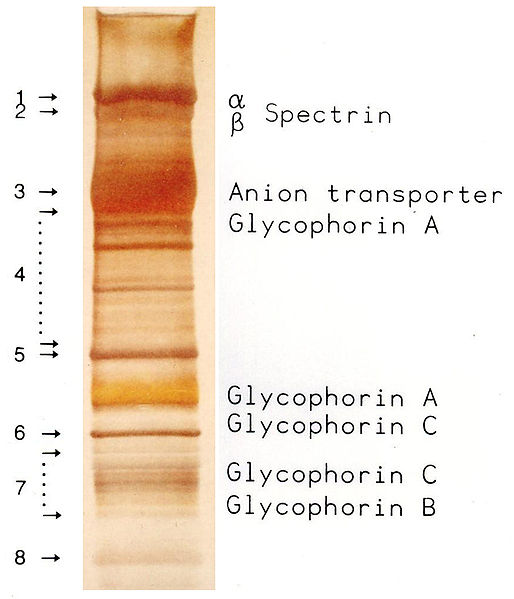
SDSPAGE patterns of different varieties of proteins. Abbreviations
When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. It combines the denaturing properties of sodium. Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β.
6 Sds Page Gel Recipe
When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. It combines the denaturing properties of sodium.
LearnSci LabSim SDSPAGE Theory Separating Proteins by Size
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. It combines the denaturing properties of sodium. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively.
Sds Unraveling Proteins' Secrets MedShun
Sds is an anionic surfactant, which can break the hydrogen and hydrophobic bonds of proteins in the presence of reducing agents (β. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively. It combines the denaturing properties of sodium.
Sds Is An Anionic Surfactant, Which Can Break The Hydrogen And Hydrophobic Bonds Of Proteins In The Presence Of Reducing Agents (Β.
It combines the denaturing properties of sodium. When loaded onto a gel matrix and placed in an electric field, the negatively charged protein molecules migrate towards the positively.